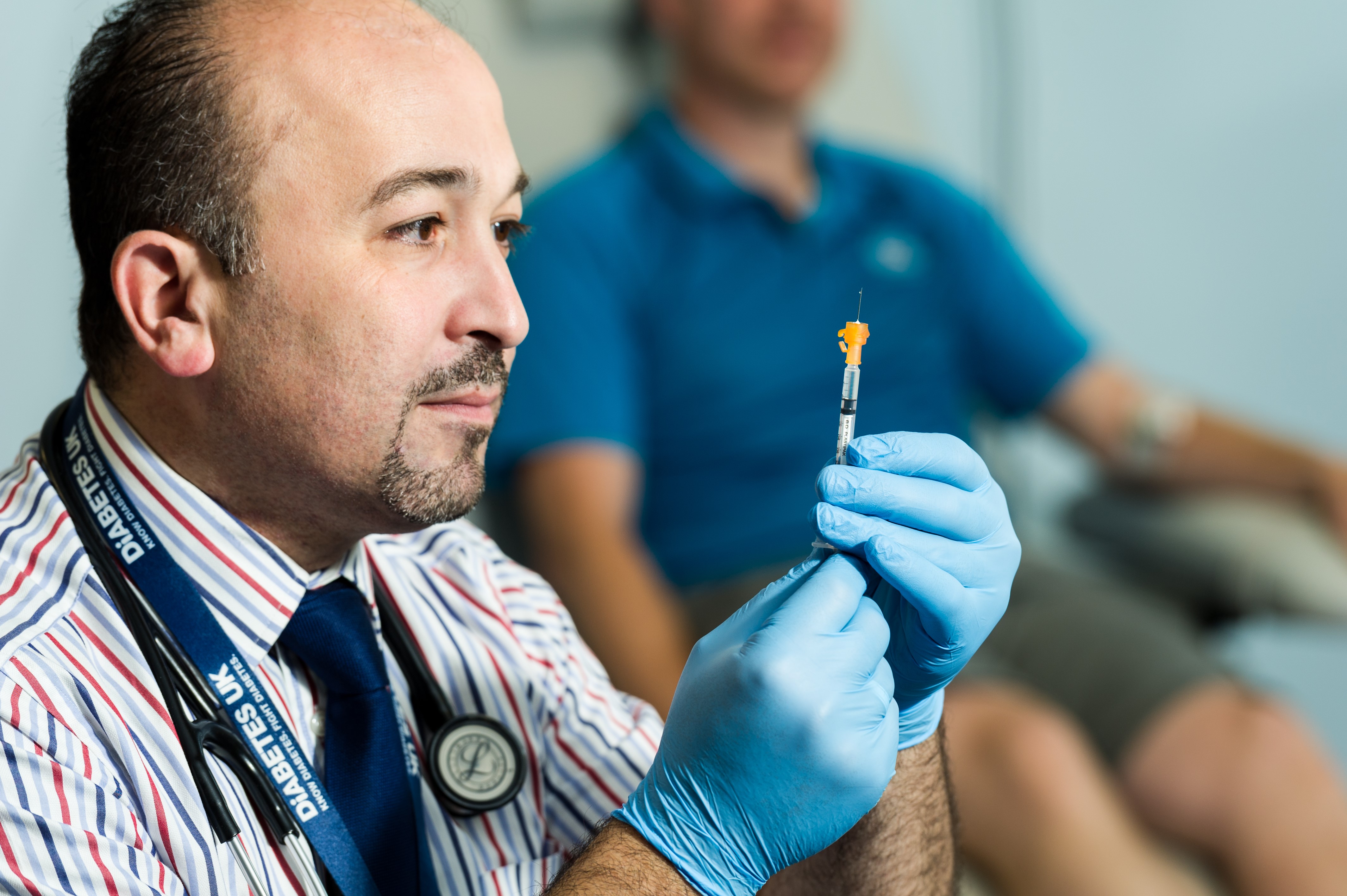
A ground-breaking clinical trial has been launched in Cardiff to help prevent and manage the chronic autoimmune disease Type 1 diabetes.
The Clinical Research Facility (CRF) at Cardiff and Vale University Health Board (UHB) has dosed the first patient in the world with the new investigational drug. The drug aims to help the regrowth of insulin making ‘beta’ cells of the pancreas, which are lost in patients living with the disease. Despite being an early phase trial, the CRF has now dosed two patients with this new drug.
Type 1 diabetes is a serious, lifelong condition where blood glucose levels are too high because the body cannot make a hormone called insulin. Those with Type 1 diabetes are dependent on insulin but if this clinical trial works, the regrowth – or regeneration – of beta cells may mean those with Type 1 diabetes becoming far less dependent on insulin injections. The benefits of the new drug would reduce lifelong conditions and complications associated with the chronic disease.
Patients who have taken part in the trial so far have spoken very highly of the treatment and the overall experience they have received at Cardiff and Vale UHB. The first patient to receive the dose said; “I’m really grateful that I was given the opportunity to take part in this study. I hope that my participation will help with the management of Type 1 diabetes for future generations.”
So far, the drug appears to have had no major side-effects, but it is too early to say if it has been effective. Cardiff’s CRF team are hoping to attract up to eight adult volunteers to take part in the clinical trial who have had diabetes for more than two years.
Dr Mohammad Alhadj Ali, who is the sub investigator working on the study in Cardiff said; “Despite everything achieved in diabetes care, advances in prevention haven’t really occurred. More insulin-producing beta cells are needed for those with this form of diabetes and it is estimated that 90% of patients with Type 1 diabetes have less than 5% of insulin making cells left.”
Professor Dayan, who is leading the team in Cardiff said, “The CRF team have made it possible to closely monitor the patients for 72 hours after their dose and I am proud of the team for their commitment in making sure this clinical trial happens as smoothly as possible.”
Dr Graham Shortland, Medical Director at Cardiff and Vale UHB said; “Cardiff and Vale UHB is committed to being a leading organisation for Research and Development in Wales, the United Kingdom and wider. This development demonstrates their commitment and the exciting work being done in our dedicated Clinical Research Facility and Cardiff and Vale more generally.”
The results of this innovative trial in diabetes treatment could be ground-breaking and is part of a wider programme of studies to preserve and regrow the insulin making cells in Type 1 diabetes being pursued by the Cardiff team, with trials in both adults and children.
Carys Thomas, interim Director of Health and Care Research Wales, said: “Developing new treatment options for patients living with diabetes is a top priority for Health and Care Research Wales. It is essential that the NHS works closely with the pharmaceutical industry on research like this to develop drugs that could make a big difference to people’s lives.
“The Clinical Research Facility in Cardiff is not only leading the way in this ground-breaking study but the team’s hard work also shows that Wales is competing successfully on an international level to attract global pharmaceutical companies and commercial investment. It will also pave the way to bring more high quality research into Wales, which could help treat other conditions.”
For more information and to get involved, please visit www.type1diabetesresearch.org.uk